Design, synthesis, biophysical and primer extension studies of novel acyclic butyl nucleic acid (BuNA)†
Abstract
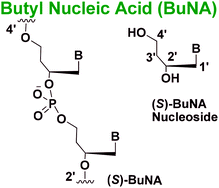
A novel nucleic acid analogue called acyclic (S)-butyl nucleic acid (BuNA) composed of an acyclic backbone containing a phosphodiester linkage and bearing natural nucleobases was synthesized. Next, (S)-BuNA nucleotides were incorporated in DNA strands and their effect on duplex stability and changes in structural conformation were investigated. Circular dichroism (CD), UV-melting and non-denatured gel electrophoresis (native PAGE) studies revealed that (S)-BuNA is capable of making duplexes with its complementary strands and integration of (S)-BuNA nucleotides into DNA duplex does not alter the B-type-helical structure of the duplex. Furthermore, (S)-BuNA oligonucleotides and (S)-BuNA substituted DNA strands were studied as primer extensions by DNA polymerases. This study revealed that the acyclic scaffold is tolerated by enzymes and is therefore to some extent biocompatible.

 Please wait while we load your content...
Please wait while we load your content...