Synthesis and biological activity of divalent ligands based on 3-deoxy-4-thiolactose, an isosteric analogue of lactose†
Abstract
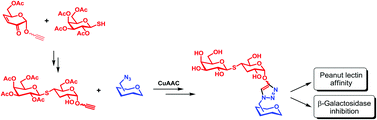
We report here the synthesis of divalent ligands containing 3-deoxy-4-thiolactose. This thiodisaccharide has been synthesized using the Michael addition of β-1-thiogalactose to the α,β-unsaturated system of sugar-derived dihydropyranones, followed by the reduction of the remaining carbonyl group. We were able to control the configuration (S) of the stereocenter linked to sulfur (C-4) of the reducing end by conducting the thioglycosylation at high temperature or by isomerization during the reduction of the 2-ulose thiodisaccharide with NaBH4/THF. The energy profile for this reaction on a model compound was calculated. The anomeric position of the 3-deoxy-4-thiolactose was functionalized with a terminal alkyne, which was coupled to azide-containing sugar scaffolds through CuAAC reaction to afford mono- and divalent ligands. The final products were competitive inhibitors of E. coli β-galactosidase in the micromolar range. Their binding affinities to peanut agglutinin (PNA) were determined by isothermal calorimetry, which showed a clear decrease in the Ka values for monovalent derivatives compared to lactose. This report contributes to establishing the role of a particular hydroxyl group of lactose in sugar-protein recognition processes.

 Please wait while we load your content...
Please wait while we load your content...