Synthesis and biological evaluation of truncated α-tubulin-binding pironetin analogues lacking alkyl pendants in the side chain or the dihydropyrone ring†
Abstract
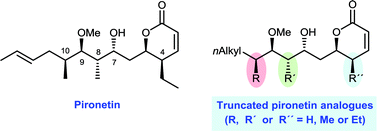
The preparation of several new truncated analogues of the natural dihydropyrone pironetin is described. They differ from the natural product mainly in the suppression of some of the alkyl pendants in either the side chain or the dihydropyrone ring. Their cytotoxic activity and their interactions with tubulin have been investigated. It has been found that all analogues are cytotoxic towards two either sensitive or resistant tumoral cell lines with similar IC50 values in each case, thus strongly suggesting that, like natural pironetin, they also display a covalent mechanism of action. Their cytotoxicity is, however, lower than that of the parent compound. This indicates that all alkyl pendants are necessary for the full biological activity, with the ethyl group at C-4 seemingly being particularly relevant. Most likely, the alkyl groups cause a restriction in the conformational mobility of the molecule and reduce the number of available conformations. This makes it more probable that the molecule preferentially adopts a shape which fits better into the binding point in α-tubulin.

 Please wait while we load your content...
Please wait while we load your content...