Synthesis of the decalin core of codinaeopsin via an intramolecular Diels–Alder reaction†
Abstract
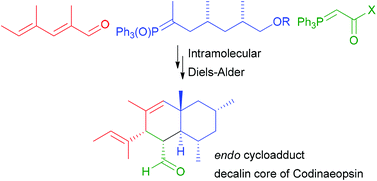
We describe the synthesis of the decalin core of codinaeopsin (1), a tryptophan-polyketide hybrid natural product with promising antimalarial activity (IC50 4.7 μM, against Plasmodium falciparum), via an intramolecular Diels–Alder (IMDA) reaction. A convergent synthesis was developed to prepare the precursors for the IMDA reaction in 10 steps. The exo cycloadducts were derived from thermal, IMDA reactions of the substrates containing a Weinreb amide or ester conjugated dienophile, and the endo adducts were from Lewis acid promoted reactions of the substrates with a formyl group. Both exo and endo products of the IMDA were exclusively isolated and characterized by NMR spectroscopy. One endo cycloadduct was further confirmed with X-ray crystallography. Theoretical calculations reveal the influence of the substituents of the decalin core on the IMDA process.

 Please wait while we load your content...
Please wait while we load your content...