A structure-based design of new C2- and C13-substituted taxanes: tubulin binding affinities and extended quantitative structure–activity relationships using comparative binding energy (COMBINE) analysis†
Abstract
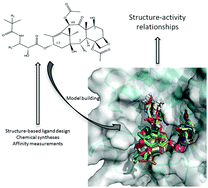
Ten novel taxanes bearing modifications at the C2 and C13 positions of the baccatin core have been synthesized and their binding affinities for mammalian tubulin have been experimentally measured. The design strategy was guided by (i) calculation of interaction energy maps with carbon,

 Please wait while we load your content...
Please wait while we load your content...