Hybrid photoactive fullerene derivative–ruboxyl nanostructures for photodynamic therapy†
Abstract
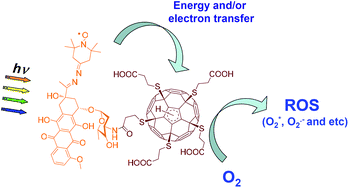
Here we report the investigation of photophysical properties and photodynamic action of two novel water soluble hybrid molecular structures based on [60]fullerene dyads bearing covalently attached residues of

 Please wait while we load your content...
Please wait while we load your content...