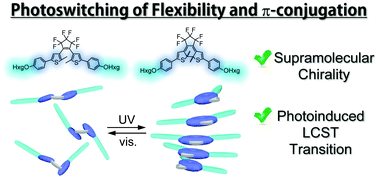
Photochromic diarylethene is a promising candidate not only for optical memory and switching units in molecular devices, but also as a photoresponsive building block that can regulate supramolecular architectures because of its characteristic changes in the flexibility of the framework and delocalization of π-electrons, along with its photoisomerization ability. Amphiphilic photochromic compounds with oligo-(ethylene glycol) side chains show good solubility in water, and self-assemble into nanostructures in aqueous media. Asymmetric introduction of a methyl group can induce a helical preference in the supramolecular structure, and the photoswitching behaviour of supramolecular chirality was defined by circular dichroism (CD) spectroscopy and density functional theory (DFT) calculations. The effective position of an asymmetric group to induce supramolecular chirality is also discussed. In addition, the self-assemblies of diarylethene derivatives in water show photoresponsive lowest critical solution temperature (LCST). The open- and closed-ring isomers show LCST transitions at different temperatures. In particular, the difference in LCST becomes prominent when amide groups are introduced at the root of hydrophilic side chains. This is attributed to intermolecular hydrogen bonds formed by the amide groups in the hydrophobic region of the supramolecular structure in water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...