Pteridine-, thymidine-, choline- and imidazole-derived alkaloids from the Australian ascidian, Leptoclinides durus†
Abstract
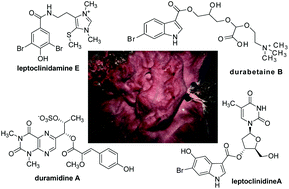
Four new acylated pteridine alkaloids, duramidines A–D, two new acylated thymidine alkaloids, leptoclinidines A and B, two new 1-acylglyceryl-3-(O-carboxyhydroxymethylcholine) alkaloids, durabetaines A and B, three new 1,3-dimethyl-5-methylsulfanylimidazole alkaloids, leptoclinidamines D–F, and the known alkaloids leptoclinidamines B and C and 6-bromo-1H-indolo-3-yl-oxoacetic acid methyl ester were isolated from the Australian ascidian Leptoclinides durus. The duramidines are the first pteridine alkaloids, possessing a three carbon side chain esterified at C-1′ with a 4-hydroxy-2′-methoxycinnamic acid, and are either hydroxylated or sulfated at C-2′. The leptoclinidines are the first 3′-indole-3-carboxylic acid ester derivatives of thymidine to be reported in the literature. The durabetaines are the first glyceryl-3-(O-carboxyhydroxymethylcholine) alkaloids to be reported from an animal source and are also the only known derivatives from this class to be acylated with aromatic carboxylic acids. MS and NMR data analysis established the structures of the new compounds. All compounds were shown to be inactive when tested for cytotoxic activity against prostate (LNCaP) and breast (MDA-MB-231) cancer cell lines and antimicrobial activity against Pseudomonas aeruginosa and Staphylococcus aureus.

 Please wait while we load your content...
Please wait while we load your content...