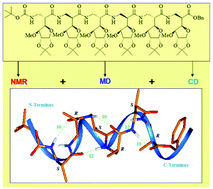
C-linked carbo-β2-amino acids (β2-Caa), a new class of β-amino acid with a carbohydrate side chain having D-xylo configuration, were prepared from D-glucose. The main idea behind the design of the new β-amino acids was to move the steric strain of the bulky carbohydrate side chain from the Cβ- to the Cα-carbon atom and to explore its influence on the folding propensities in peptides with alternating (R)- and (S)-β2-Caas. The tetra- and hexapeptides derived were studied employing NMR (in CDCl3), CD, and molecular dynamics simulations. The β2-peptides of the present study form left-handed 12/10- and 10/12-mixed helices independent of the order of the alternating chiral amino acids in the sequence and result in a new motif. These results differ from earlier findings on β3-peptides of the same design, containing a carbohydrate side chain with D-xylo configuration, which form exclusively right-handed 12/10-mixed helices. Quantum chemical calculations employing ab initio MO theory suggest the side chain chirality as an important factor for the observed definite left- or right-handedness of the helices in the β2- and β3-peptides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...