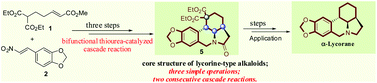
Concise construction of the tetracyclic core of lycorine-type alkaloids and the formal synthesis of α-lycorane based on asymmetric bifunctional thiourea-catalyzed cascade reaction†
Abstract
A concise and stereoselective construction of the tetracyclic core of lycorine-type

 Please wait while we load your content...
Please wait while we load your content...