Asymmetric synthesis of a highly functionalized enantioenriched system close to thapsigargin framework†
Abstract
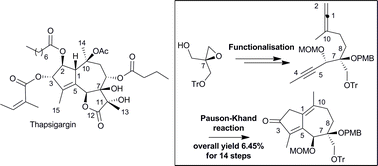
A straightforward approach to a highly functionalized enantioenriched bicyclo[5.3.0]decadienone system close to the thapsigargin framework has been achieved. The developed synthetic route involves two main stages: installation of the chains on either side of the quaternary center at C7 starting from a central enantiopure

 Please wait while we load your content...
Please wait while we load your content...