Enantioselective allylation of imines catalyzed by newly developed (−)-β-pinene-based π-allylpalladium catalyst: an efficient synthesis of (R)-α-propylpiperonylamine and (R)-pipecolic acid†‡
Abstract
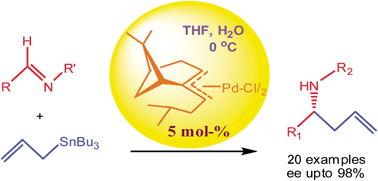
A newly developed π-allylpalladium with a (−)-β-pinene framework and an

 Please wait while we load your content...
Please wait while we load your content...