Between two worlds: a comparative study on in vitro and in silico inhibition of trypsin and matriptase by redox-stable SFTI-1 variants at near physiological pH†
Abstract
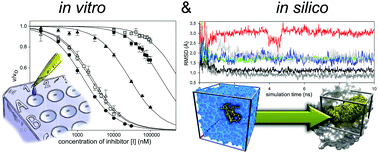
A comparative study on in vitro and in silico inhibition of trypsin and matriptase by derivatives of the sunflower trypsin inhibitor-1 at near physiological pH is reported. Besides wild-type bicyclic SFTI-1, monocyclic variants possessing native

 Please wait while we load your content...
Please wait while we load your content...