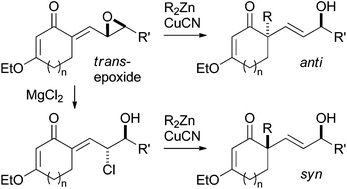
We developed a new method for stereoselective construction of an all-carbon quaternary stereogenic center on a carbocyclic ring based on regio- and stereoselective SN2′ alkylation reactions of γ,δ-epoxy-α,β-unsaturated cyclic ketones. Treatment of the ketones, which were readily prepared in enantiomerically pure form by means of aldol condensations between 3-ethoxy-2-cycloalkenones and α,β-epoxy aldehydes, with a R2Zn–CuCN reagent afforded anti-SN2′ products stereoselectively. Conversely, the corresponding syn-SN2′ products were stereoselectively obtained through two-step transformations of the same γ,δ-epoxy-α,β-unsaturated cyclic ketones: (1) conversion of the epoxide moiety to a chlorohydrin by treatment with MgCl2 and (2) subsequent SN2′ substitution of the chlorohydrin with a R2Zn–CuCN reagent. These substitution products with their chiral trans-allylic alcohol moieties are promising precursors for complex molecules. For example, Eschenmoser–Claisen rearrangement of one of the substitution products resulted in stereoselective formation of a keto amide having contiguous quaternary and tertiary stereogenic centers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...