Total synthesis of dendrobate alkaloid (+)-241D, isosolenopsin and isosolenopsin A: application of a gold-catalyzed cyclization†
Abstract
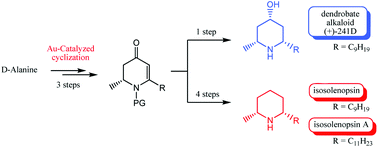
A new approach to total syntheses of piperidine alkaloids (+)-241D, isosolenopsin and isosolenopsin A has been developed from D-alanine. The key step to access the chiral pyridinone intermediate was achieved via a gold mediated cyclization. Finally, various reduction conditions afforded the natural products in few steps and good overall yields.

 Please wait while we load your content...
Please wait while we load your content...