Theoretical study on the mechanism of Ag-catalyzed synthesis of 3-alkylideneoxindoles from N-aryl-α-diazoamides: a Lewis acid or Ag-carbene pathway?†
Abstract
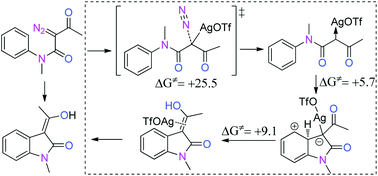
The mechanism of the title reaction is found to consist of three steps by DFT calculations: (1) N2 dissociation, (2) intramolecular Ag-carbene addition, and (3)

 Please wait while we load your content...
Please wait while we load your content...