A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: co-formulation opportunities for siRNA delivery†
Abstract
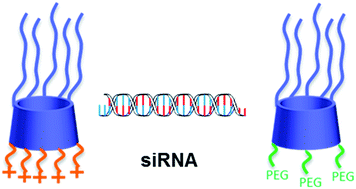
A new approach to the synthesis of amphiphilic β-cyclodextrins has used ‘click’ chemistry to selectively modify the secondary 2-hydroxyl group. The resulting extended polar groups can be either polycationic or neutral PEGylated groups and these two amphiphile classes are compatible in dual cyclodextrin formulations for delivery of siRNA. When used alone with an siRNA, a cationic cyclodextrin was shown to have good transfection properties in cell culture. Co-formulation with a PEGylated cyclodextrin altered the physicochemical properties of nanoparticles formed with siRNA. Improved particle properties included lower surface charges and reduced tendency to aggregate. However, as expected, the transfection efficiency of the cationic vector was lowered by co-formulation with the PEGylated cyclodextrin, requiring future surface modification of particles with targeting ligands for effective siRNA delivery.
 Please wait while we load your content...
Please wait while we load your content...