A new approach to construct a fused 2-ylidene chromene ring: highly regioselective synthesis of novel chromeno quinoxalines†
Abstract
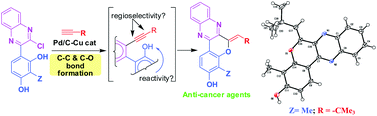
Regioselective construction of a fused 2-ylidene chromene ring was achieved for the first time by using AlCl3-induced C–C bond formation followed by Pd/C–Cu mediate coupling-

 Please wait while we load your content...
Please wait while we load your content...