Synthesis of carbazolones and 3-acetylindoles via oxidative C–N bond formation through PIFA-mediated annulation of 2-aryl enaminones†
Abstract
A series of carbazolone derivatives and 3-acetylindoles have been achieved via PIFA-mediated intramolecular
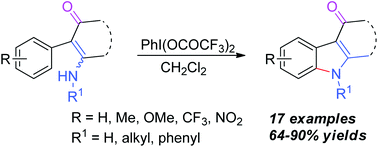

 Please wait while we load your content...
Please wait while we load your content...