Phosphine-mediated cascade reaction of azides with MBH-acetates of acetylenic aldehydes to substituted pyrroles: a facile access to N-fused pyrrolo-heterocycles†
Abstract
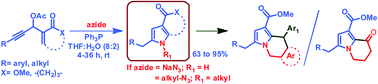
One-pot synthesis of substituted pyrroles by a cascade reaction of azides with Morita–Baylis–Hillman acetates of acetylenic aldehydes is described and the reaction is efficiently mediated by triphenyl phosphine at room temperature. Sodium azide is successfully used to provide N-unsubstituted pyrroles, while alkyl azides afforded the corresponding N-alkylated pyrroles through a sequence of allylic substitution/azide reduction/cycloisomerization reactions. The obtained products have provided a new entry to indolizino indoles, pyrrolo isoquinolines and 8-oxo-5,6,7,8-tetrahydroindolizine.
 Please wait while we load your content...
Please wait while we load your content...