Synthesis of amino-substituted indoles using the Bartoli reaction†
Abstract
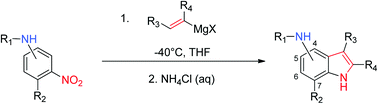
We report herein the concise preparation of a range of functionalised aminoindoles via a new application of the Bartoli reaction. Scope and limitations of the methodology have been extensively studied to reveal the importance of protecting groups and substitution patterns. The use of
 Please wait while we load your content...
Please wait while we load your content...