Chemoselective Staudinger-phosphite reaction of symmetrical glycosyl-phosphites with azido-peptides and polygycerols†‡
Abstract
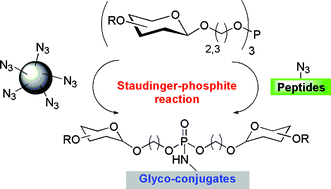
In this paper we present the synthesis of glyco-phosphoramidate conjugates as easily accessible analogs of glyco-phosphorous
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...