Conformational flexibility of the pentasaccharide LNF-2 deduced from NMR spectroscopy and molecular dynamics simulations†
Abstract
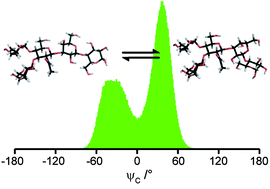
Human milk oligosaccharides (HMOs) are important as prebiotics since they stimulate the growth of beneficial bacteria in the intestine and act as receptor analogues that can inhibit the binding of pathogens. The conformation and dynamics of the HMO Lacto-N-fucopentaose 2 (LNF-2), α-L-Fucp-(1 → 4)[β-D-Galp-(1 → 3)]-β-D-GlcpNAc-(1 → 3)-β-D-Galp-(1 → 4)-D-Glcp, having a Lewis A epitope, has been investigated employing NMR spectroscopy and molecular dynamics (MD) computer simulations. 1D 1H,1H-NOESY experiments were used to obtain proton–proton cross-relaxation rates from which effective distances were deduced and 2D J-HMBC and 1D long-range experiments were utilized to measure trans-glycosidic 3JCH coupling constants. The MD simulations using the PARM22/SU01 force field for carbohydrates were carried out for 600 ns with explicit water as solvent which resulted in excellent sampling for flexible glycosidic torsion angles. In addition, in vacuo MD simulations were performed using an MM3-2000 force field, but the agreement was less satisfactory based on an analysis of heteronuclear trans-glycosidic coupling constants. LNF-2 has a conformationally well-defined region consisting of the terminal branched part of the pentasaccharide, i.e., the Lewis A epitope, and a flexible β-D-GlcpNAc-(1 → 3)-β-D-Galp-linkage towards the lactose unit, which is situated at the reducing end. For this β-(1 → 3)-linkage a negative ψ torsion angle is favored, when experimental NMR data is combined with the MD simulation in the analysis. In addition, flexibility on a similar time scale, i.e., on the order of the global overall molecular reorientation, may also be present for the ϕ torsion angle of the β-D-Galp-(1 → 4)-D-Glcp-linkage as suggested by the simulation. It was further observed from a temperature variation study that some 1H NMR chemical shifts of LNF-2 were highly sensitive and this study indicates that Δδ/ΔT may be an additional tool for revealing conformational dynamics of oligosaccharides.

 Please wait while we load your content...
Please wait while we load your content...