Palladium(ii) acetate mediated oxidative cyclization of ω-unsaturated α-cyano ketones for facile construction of methylenecyclohexane ring system†
Abstract
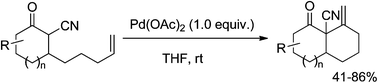
A highly efficient annulative approach towards the construction of the structurally attractive methylenecyclohexane ring was developed through a convenient 1,4-addition of

 Please wait while we load your content...
Please wait while we load your content...