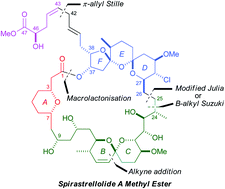
The spirastrellolides are a novel family of structurally unprecedented marine macrolides which show promising anticancer properties due to their potent inhibition of protein phosphatase 2A. In the preceding paper, a modular strategy for the synthesis of spirastellolide A methyl ester which allowed for the initial stereochemical uncertainties was outlined, together with the synthesis of a series of suitably functionalised fragments. In this paper, the realisation of this synthesis is described. Two alternative coupling strategies were explored for elaborating the C26–C40 DEF bis-spiroacetal fragment: a modified Julia olefination of a C26 aldehyde with a C17–C25 sulfone, and a Suzuki coupling of a C25 trialkylborane with a C17–C24 vinyl iodide, which also required the development of a double hydroboration reaction to install the C23/C24 stereocentres. The latter proved a significantly superior strategy, and was fully optimised to provide a C17 aldehyde which was coupled with a C1–C16 alkyne fragment to afford the C1–C40 carbon framework. The BC spiroacetal was then installed within this advanced intermediate by oxidative cleavage of two PMB ethers with spontaneous spiroacetalisation, which also led to unanticipated deprotection of the C23 TES ether. The ensuing truncated seco-acid was cyclised in high yield to construct the 38-membered macrolactone under Yamaguchi macrolactonisation conditions, suggesting favourable conformational pre-organisation. Exhaustive desilylation provided a crystalline macrocyclic pentaol, revealing much about the likely conformation of the macrolactone in solution. Attachment of the remainder of the side chain proved challenging, potentially due to steric hindrance by this macrocycle; an olefin cross-metathesis to install an electrophilic allylic carbonate and subsequent π-allyl Stille coupling with a C43–C47 stannane achieved this goal. Global deprotection completed the first total synthesis of (+)-spirastrellolide A methyl ester which, following detailed NMR correlation with an authentic sample, validated the full configurational assignment. A series of simplified analogues of spirastrellolide incorporating the C26–C47 region were also prepared by π-allyl Stille coupling reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...