Iron-catalyzed ene-type propargylation of diarylethylenes with propargyl alcohols†
Abstract
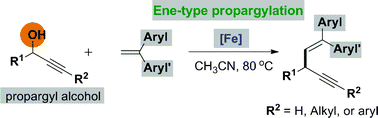
The diarylalkenyl propargylic complex framework has been found in many natural products and medicinal regents. Herein, we have disclosed an unprecedented FeCl3 catalyzed ene-type reaction of propargylic

 Please wait while we load your content...
Please wait while we load your content...