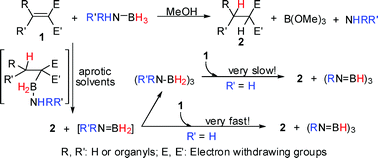
Metal-free transfer hydrogenation of polarized olefins (RR′C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CEE′: R, R′ = H or organyl, E, E′ = CN or CO2Me) using amine borane adducts RR′NH–BH3 (R = R′ = H, AB; R = Me, R′ = H, MAB; R = tBu, R′ = H, tBAB; R = R′ = Me, DMAB) as hydrogen donors, were studied by means of in situ NMR spectroscopy. Deuterium kinetic isotope effects and the traced hydroboration intermediate revealed that the double H transfer process occurred regio-specifically in two steps with hydride before proton transfer characteristics. Studies on substituent effects and Hammett correlation indicated that the rate determining step of the HN transfer is in agreement with a concerted transition state. The very reactive intermediate [NH2
CEE′: R, R′ = H or organyl, E, E′ = CN or CO2Me) using amine borane adducts RR′NH–BH3 (R = R′ = H, AB; R = Me, R′ = H, MAB; R = tBu, R′ = H, tBAB; R = R′ = Me, DMAB) as hydrogen donors, were studied by means of in situ NMR spectroscopy. Deuterium kinetic isotope effects and the traced hydroboration intermediate revealed that the double H transfer process occurred regio-specifically in two steps with hydride before proton transfer characteristics. Studies on substituent effects and Hammett correlation indicated that the rate determining step of the HN transfer is in agreement with a concerted transition state. The very reactive intermediate [NH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2] generated from AB was trapped by addition of cyclohexene into the reaction mixture forming Cy2BNH2. The final product borazine (BHNH)3 is assumed to be formed by dehydrocoupling of [NH2
BH2] generated from AB was trapped by addition of cyclohexene into the reaction mixture forming Cy2BNH2. The final product borazine (BHNH)3 is assumed to be formed by dehydrocoupling of [NH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2] or its solvent stabilized derivative [NH2
BH2] or its solvent stabilized derivative [NH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2]–(solvent), rather than by dehydrogenation of cyclotriborazane (BH2NH2)3 which is the trimerization product of [NH2
BH2]–(solvent), rather than by dehydrogenation of cyclotriborazane (BH2NH2)3 which is the trimerization product of [NH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2].
BH2].
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CEE′: R, R′ = H or
CEE′: R, R′ = H or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2] generated from AB was trapped by addition of
BH2] generated from AB was trapped by addition of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2] or its
BH2] or its ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2]–(
BH2]–(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2].
BH2].

 Please wait while we load your content...
Please wait while we load your content...