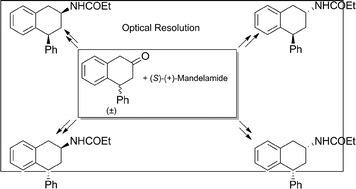
An efficient and practical approach for the synthesis of all four stereoisomers of the MT2 melatonin receptor ligand 4-phenyl-2-propionamidotetralin (4-P-PDOT), each in enantiomerically pure form (ee > 99.9%), was developed. The strategy involved an optical resolution procedure of the key precursor (±)-4-phenyl-2-tetralone with the unusual resolving agent (S)-mandelamide, through the formation of four dihydronaphtalene-spiro-oxazolidin-4-one diastereomers. Interestingly, NMR experimental observations in combination with geometric calculations, provided unambiguous configuration assignments of all stereocenters of the key spiro stereoisomers. Cleavage of each single spiro diastereomer under acidic conditions gave enantiopure (R)- or (S)-4-phenyl-2-tetralone, which were then converted to each 4-P-PDOT single enantiomer by using stereoselective reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...