Synthesis of new 9-hydroxy-α- and 7-hydroxy-β-pyran naphthoquinones and cytotoxicity against cancer cell lines†
Abstract
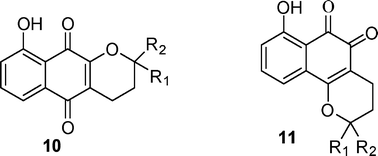
A synthetic method to obtain α- and β-pyran naphthoquinones 10 and 11 with a hydroxyl substituent on the aromatic ring was developed. Two series of α- and β-pyran

 Please wait while we load your content...
Please wait while we load your content...