Highly efficient and regiospecific photocyclization of 2,2′-diacyl bixanthenylidenes†
Abstract
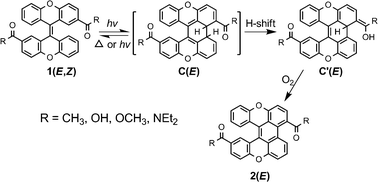
In contrast to the reversible photochemistry of the 2,2′-substituted bixanthenylidenes (1a–f), the photocyclization of 2,2′-diacyl bixanthenylidenes (1g–j) reveals an irreversible process where the initial cyclic intermediate C(E) can undergo a rapid [1,11] hydrogen shift to form stable isomer C′(E) in a degassed solution, which cannot revert to the starting compound, so giving a highly efficient and regiospecific photocyclization.

 Please wait while we load your content...
Please wait while we load your content...