A cascade process for the synthesis of gem-difluoromethylene compounds†
Abstract
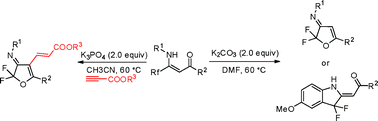
An efficient strategy for the synthesis of 2,2-difluoro-2,3-dihydrofuran derivatives from β-fluoroalkyl-β-enaminoketones is described. The reaction occurred via an intramolecular halophilic attack-initiated cascade process. A series of 2,3-dihydrofurans were prepared in high yields. And an intermolecular domino process achieved providing polysubstituted

 Please wait while we load your content...
Please wait while we load your content...