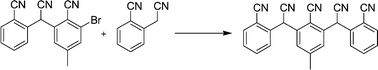
Highly electron deficient monoaryl, di-aryl and bis-diaryl acetonitriles were effectively synthesized using either a nucleophilic aromatic substitution (NAS) or a palladium-mediated coupling pathway. Synthesis of di-aryl acetonitriles most conveniently proceeded via NAS – palladium-mediated coupling was not required. This reaction, however, results in a product that is more acidic than the reactants. Facile deprotonation of the product prevents efficient formation of the bis-diaryl acetonitrile through a NAS pathway. Thus, palladium-mediated coupling is required to prepare the bis-diaryl acetonitrile efficiently. In the palladium-catalyzed coupling, choice of base and solvent (and thus the counter cation for the benzylic anion nucleophile) is important. Also, choice of the supporting ligand is important, indicating the sensitivity of the reaction to steric and ligand electronic effects.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...