Enantiomerically pure 2-aryl(alkyl)-2-trifluoromethylaziridines: synthesis and ring opening with selected O- and N-nucleophiles†‡
Abstract
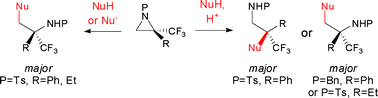
We report herein the synthesis of enantiomerically pure 2-phenyl- and 2-ethyl-2-trifluoromethylaziridines by Mitsunobu-type cyclisation of the corresponding N-protected

 Please wait while we load your content...
Please wait while we load your content...