Convergent assembly of structurally diverse quinazolines†
Abstract
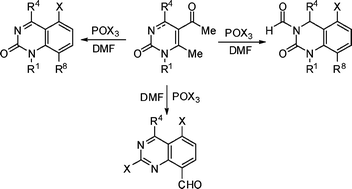
A convergent and versatile Vilsmeier–Haack-based carbo-annulation strategy that exhibits an unusually elevated bond-forming efficiency has been developed. By virtue of its innovative approach, structure economy and simple execution conditions the methodology reported here constitutes a very attractive protocol that enables the rapid assembly of structurally diverse

 Please wait while we load your content...
Please wait while we load your content...