A previous report that the interstellar molecule glycolaldehyde (HOCH2CHO) can be made from hydroxymethylene (HOCH:) and formaldehyde has been revisited at the CCSD(T)/6-311++G(3df,2p)//MP2/6-311++G(3df,2p) level of theory. This reaction competes with the formation of acetic acid and methylformate, molecules which have also been detected in interstellar clouds. Other possible modes of formation of glycolaldehyde by radical/radical reactions have been shown to be viable theoretically as follows:HO˙ + ˙CH2CHO → HOCH2CHO [ΔGr(298 K) = −303 kJ mol−1]HOCH2˙ + ˙CHO → HOCH2CHO (−259 kJ mol−1)
The species in these two processes are known interstellar molecules. Key radicals ˙CH2CHO and ˙CH2OH in these sequences have been shown to be stable for the microsecond duration of neutralization/reionization experiments in the dual collision cells of a VG ZAB 2HF mass spectrometer. The polymerization reaction HOCH2CH˙OH + nCH2O → HOCH2[CH(OH)]n˙CHOH (n = 1 to 3) has been studied theoretically and shown to be energetically feasible, as is the cyclization reaction of HOCH2[(CH2OH)4]˙CHOH (in the presence of one molecule of water at the reacting centre) to form glucose. The probability of such a reaction sequence is small even if polymerization were to occur in interstellar ice containing a significant concentration of CH2O. The large number of stereoisomers produced by such a reaction sequence makes the formation of a particular sugar, again for example glucose, an inefficient synthesis. The possibility of stereoselectivity occurring during the polymerization was investigated for two diastereoisomers of HOCH2[(CHOH)]2˙CHOH. No significant difference was found in the transition state energies for addition of CH2O to these two diastereoisomers, but a barrier difference of 12 kJ mol−1 was found for the H transfer reactions ˙OCH2[(CHOH)]2CH2OH → HOCH2[(CHOH)2˙CHOH of the two diastereoisomers.
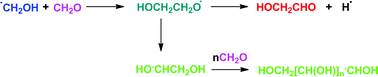
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
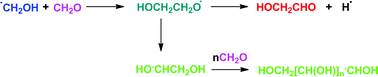

 Please wait while we load your content...
Please wait while we load your content...