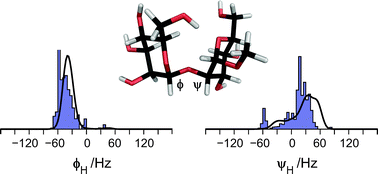
The conformational space available to the flexible molecule α-D-Manp-(1→2)-α-D-Manp-OMe, a model for the α-(1→2)-linked mannose disaccharide in N- or O-linked glycoproteins, is determined using experimental data and molecular simulation combined with a maximum entropy approach that leads to a converged population distribution utilizing different input information. A database survey of the Protein Data Bank where structures having the constituent disaccharide were retrieved resulted in an ensemble with >200 structures. Subsequent filtering removed erroneous structures and gave the database (DB) ensemble having three classes of mannose-containing compounds, viz., N- and O-linked structures, and ligands to proteins. A molecular dynamics (MD) simulation of the disaccharide revealed a two-state equilibrium with a major and a minor conformational state, i.e., the MD ensemble. These two different conformation ensembles of the disaccharide were compared to measured experimental spectroscopic data for the molecule in water solution. However, neither of the two populations were compatible with experimental data from optical rotation, NMR 1H,1H cross-relaxation rates as well as homo- and heteronuclear 3J couplings. The conformational distributions were subsequently used as background information to generate priors that were used in a maximum entropy analysis. The resulting posteriors, i.e., the population distributions after the application of the maximum entropy analysis, still showed notable deviations that were not anticipated based on the prior information. Therefore, reparameterization of homo- and heteronuclear Karplus relationships for the glycosidic torsion angles ϕ and ψ were carried out in which the importance of electronegative substituents on the coupling pathway was deemed essential resulting in four derived equations, two 3JCOCC and two 3JCOCH being different for the ϕ and ψ torsions, respectively. These Karplus relationships are denoted JCX/SU09. Reapplication of the maximum entropy analysis gave excellent agreement between the MD- and DB-posteriors. The information entropies show that the current reparametrization of the Karplus relationships constitutes a significant improvement. The ϕH torsion angle of the disaccharide is governed by the exo-anomeric effect and for the dominating conformation ϕH = −40° and ψH = 33°. The minor conformational state has a negative ψH torsion angle; the relative populations of the major and the minor states are ∼3 : 1. It is anticipated that application of the methodology will be useful to flexible molecules ranging from small organic molecules to large biomolecules.

 Please wait while we load your content...
Please wait while we load your content...