Asymmetric Michael addition reaction of 3-substituted-N-Boc oxindoles to activated terminal alkenes catalyzed by a bifunctional tertiary-amine thiourea catalyst†
Abstract
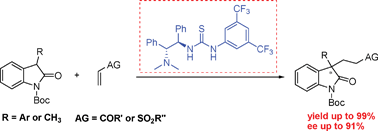
The current article reports an organocatalytic strategy for the asymmetric catalysis of chiral oxindoles bearing 3-position all-

 Please wait while we load your content...
Please wait while we load your content...