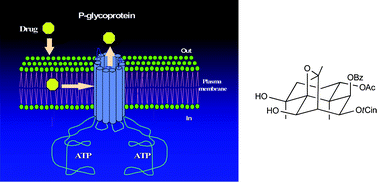
P-Glycoprotein (Pgp) overexpression is one factor contributing to multidrug resistance (MDR) in cancer cells and represents one drawback in the treatment of cancer. In an attempt to find more specific and less toxic anticancer MDR-reversal agents, we report herein the isolation, structure elucidation and biological activity of nine new (1–5, 9–11 and 13) and seven known (6–8, 12 and 14–16) dihydro-β-agarofuran sesquiterpenes from the leaves of Celastrus vulcanicola. Their stereostructures were elucidated on the basis of spectroscopic analysis, including 1D and 2D NMR techniques, CD studies and biogenetic means. All the compounds were assayed on human MDR1-transfected NIH-3T3 cells, in order to determine their ability to reverse the MDR phenotype due to Pgp overexpression. Six compounds from these series (3, 5, 6, 9, 11 and 12) showed an effectiveness that was similar to (or higher than) the classical Pgp reversal agent verapamil for the reversal of resistance to daunomycin and vinblastine. The structure–activity relationships are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...