Aryl tert-butyl sulfoxide-promoted highly enantioselective addition of allyltrichlorosilane to aldehydes†
Abstract
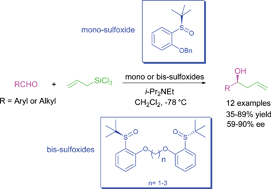
A series of enantiomerically pure mono- and bis-aryl tert-butyl sulfoxides were synthesised to promote the enantioselective allylation of

 Please wait while we load your content...
Please wait while we load your content...