Fluoride recognition by a chiral urea receptor linked to a phthalimide chromophore†
Abstract
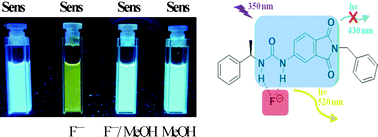
The anion chemosensor 1 based on a urea-activated phthalimide with a stereogenic centre was synthesized using an efficient procedure involving a Curtius rearrangement. Its photophysical properties were estimated in several

 Please wait while we load your content...
Please wait while we load your content...