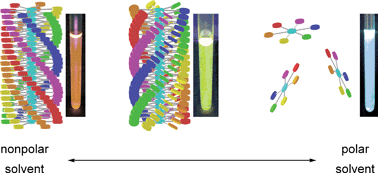
A C6-symmetric disk-like molecule, a hexakis(phenylethynyl)benzene derivative bearing chiral alanine parts, L-1, exhibited a solvent-induced supramolecular helix-sense inversion involving conformational changes followed by destruction of the supramolecular helical column. This phenomenon has been found by investigating the supramolecular assembly state of L-1 in mixed solvents of various chloroform (CHCl3)/n-hexane (Hx) ratios. L-1 forms a stable helical columnar assemblyvia multiple noncovalent bonding interactions in nonpolar Hx, while the molecules in relatively polar CHCl3 are in a molecularly dispersed state. Although one would expect disruption of the helical column with the addition of nonhelicogenic CHCl3, an opposite-handed helical columnar structure was formed at 8–15 vol% of CHCl3, and subsequently the inverted helical column was disassembled by a further increase of CHCl3. In addition, this morphological transformation was accompanied by a significant change in fluorescent color, which varies over a wide visible range from orange in an original helical columnar state to light blue in a molecularly dispersed state through yellow in an inverted helical columnar state. These unprecedented behaviors are shown by the spectroscopic results, and the molecular conformations of L-1 and the driving force for the helical sense inversion are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...