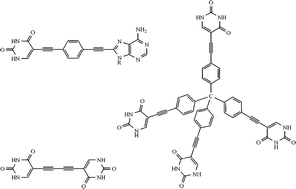
Being aimed at a new type of porous solids, a moduled design strategy of molecular tectons, making use of the conjugation between a shape defined artificial backbone and the bioinspired molecular fragments of nucleobases or nucleobase derivatives as functional end-caps, has been developed. This led to the formation of the new hybrid compounds 1–13 of linear and tetrahedral geometry, containing uracil, adenine, adenosine, guanosine and its acylated analogs as the sticky end-cap sites. The compounds were synthesized from a halogen or ethynyl substituted nucleobase component and the corresponding ethynylated spacer unit following a metal assisted coupling process as the key reaction step. X-Ray crystal structure analysis demonstrates that the parent compound 1 is a solvent complex with DMSO (1:2), showing the DMSO molecules incorporated in a hydrogen bonded layer structure. Specific dependencies of the fluorescence properties of the new compounds in solution on the structure of the molecules are reported. A selection of solid compounds has been studied in respect of their ability to adsorb organic vapours. They revealed significant differences both in the sorption capacity and the selectivity towards particular solvent vapours.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...