A versatile approach to oligostilbenoid natural products – synthesis of permethylated analogues of viniferifuran, malibatol A, and shoreaphenol†
Abstract
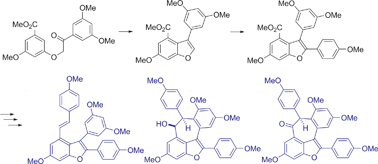
A highly practical route to oligostilbenoid natural products is described. A regioselective Bi(OTf)3-catalyzed cyclodehydration provided ready access to 3-arylbenzofuran. Pd-catalyzed direct C–H

 Please wait while we load your content...
Please wait while we load your content...