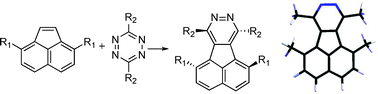
The synthesis of a series of 3,6-disubstituted-1,2,4,5-tetrazines has been effected using an inverse electron demand [2 + 4] cycloaddition strategy. The crystal structures of 18 members of this series of diazafluoranthenes are reported. Stereochemical analysis shows that diazafluoranthenes, substituted across the bay region, are helically-twisted strained aromatic molecules. The dihedral angle between pyridazylvsnaphthyl rings ranges from 0.5° to 20.9°, and follows the degree of steric congestion in the bay region. The crystal structures are compared to computational structures determined using density functional theory, with the M06-2X/cc-pVDZ method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...