Tandem copper-catalysed aryl and alkenyl amination reactions: the synthesis of N-functionalised indoles†
Abstract
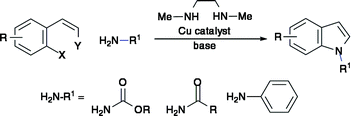
A Cu-diamine complex effectively catalyses tandem C–N bond formation on 2-(2-haloalkenyl)-aryl halide substrates, to deliver a series of N-functionalised

 Please wait while we load your content...
Please wait while we load your content...