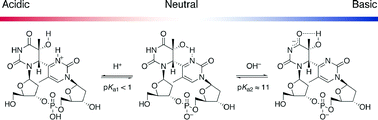
We synthesized a dinucleoside monophosphate of the 15N-labeled (6–4) photoproduct, which is one of the major UV-induced lesions in DNA, to investigate the (6–4) photolyase repair mechanism, and characterized its protonation state by measuring 15N NMR spectra as a function of pH. We expected that chemical-shift changes of the pyrimidone15N3, due to protonation, would be observed at pH 3, as observed for the 15N-labeled 5-methylpyrimidin-2-one nucleoside. Interestingly, however, the changes were observed only in alkaline solutions. In UV absorption spectroscopy and HPLC analyses under acidic conditions, a change in the maximum absorption wavelength, due to the protonation-induced hydrolysis, was observed at and below pH 1, but not at pH 2, whereas the protonation of 5-methylpyrimidin-2-one occurred at pH values between 2 and 3. These results indicated that the pKa value for this N3 is remarkably lower than that of a normal pyrimidone ring, and strongly suggest that an intramolecular hydrogen bond is formed between the N3 of the 3′ base and the 5-OH of the 5′ base under physiological conditions. The results of this study have implications not only for the recognition and reaction mechanisms of (6–4) photolyase, but also for the chemical nature of the (6–4) photoproduct.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...