New kinds of organic superacids. Bis(perfluoroalkylsulfonylimino)trifluoromethanesulfonic acids and their trimethylsilyl esters†
Abstract
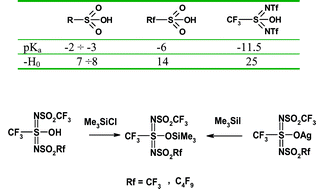
A synthesis of new unsymmetric trifluoromethylsulfonylimino(nonafluoro-n-butyl-sulfonylimino)trifluoromethanesulfonic acid (1b) is reported. During the silylation reactions studies of bis(perfluoroalkylsulfonylimino)trifluoromethanesulfonic acids (1a,b) it was shown that reaction proceeds exclusively on the central oxygen atom to give trimethylsilyl esters. The obtained

 Please wait while we load your content...
Please wait while we load your content...