Highly diastereoselective desymmetrisation of cyclic meso-anhydrides and derivatisation for use in natural product synthesis†
Abstract
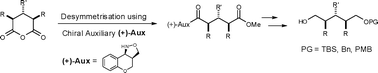
A new and efficient desymmetrisation of succinic and glutaric cyclic meso-anhydrides is described, providing excellent yields and diastereoselectivities in most cases.

 Please wait while we load your content...
Please wait while we load your content...