Effect of (5′S)-5′,8-cyclo-2′-deoxyadenosine on the conformation of di and trinucleotides. A NMR and DFT study
Abstract
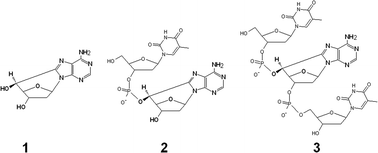
5′,8-Purine cyclonucleosides constitute an important class of oxidatively generated tandem lesions whose formation involves initial

 Please wait while we load your content...
Please wait while we load your content...