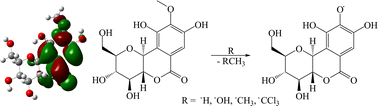
(+)-Bergenin (1) was isolated from Sacoglottis uchi, a species of vegetable found in the Amazon region and popularly used for the treatment of several hepatic problems. The structure of 1 was fully characterized using IR, GC–MS and NMR (1D and 2D) analyses. This phytoconstituent has been used as an oriental folk medicine for the treatment of many diseases and shows antihepatotoxic properties. Tests with β-carotene, DPPH and a heterogeneous Fenton system were carried out, confirming the antioxidant activity of 1. Theoretical calculations were performed to investigate the formation of the radical derivatives of 1 using ˙H, ˙OH, ˙CH3, and ˙CCl3 as initiator radicals. DFT thermodynamic calculations showed that the methoxyl group (O-6–CH3) is the most favorable site for radical attack. Frontier molecular orbital analysis showed that nucleophilic radical attack is favored on the aromatic ring of 1 where the LUMO is localized, with antibonding character with respect to the O-6–CH3 bond. The possibilities of attack at other sites on 1 were investigated in detail in order to understand the regiospecificity of this reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...